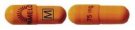Intended for U.S. healthcare professionals only.
For corporate information, please visit Mallinckrodt.com

In This Section
- Generic Products
- Addiction Treatment Products
- Compounding Powders
-
Non-promoted Brands
-
Central Nervous System Products
- Anafranil™ (clomipramine hydrochloride) Capsules, USP 25 mg
- Anafranil™ (clomipramine hydrochloride) Capsules, USP 50 mg
- Anafranil™ (clomipramine hydrochloride) Capsules, USP 75 mg
- Pamelor™ (nortriptyline HCl) Capsules, USP 10 mg
- Pamelor™ (nortriptyline HCl) Capsules, USP 25 mg
- Pamelor™ (nortriptyline HCl) Capsules, USP 50 mg
- Pamelor™ (nortriptyline HCl) Capsules, USP 75 mg
- Restoril™ (temazepam) Capsules USP, CIV 15 mg
- Restoril™ (temazepam) Capsules USP, CIV 22.5 mg
- Restoril™ (temazepam) Capsules USP, CIV 30 mg
- Restoril™ (temazepam) Capsules USP, CIV 7.5 mg
- Opioid Products
-
Central Nervous System Products
- Resource Tool Kit
- History
- Contact Generics
Pamelor™ (nortriptyline HCl) Capsules, USP 75 mg
INDICATIONS AND USAGE
Pamelor™ (nortriptyline HCl) capsules USP is indicated for the relief of symptoms of depression. Endogenous depressions are more likely to be alleviated than are other depressive states.
IMPORTANT SAFETY INFORMATION
|
Suicidality and Antidepressant Drugs Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of nortriptyline hydrochloride or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Nortriptyline hydrochloride is not approved for use in pediatric patients. |
CONTRAINDICATIONS
- Pamelor is contraindicated:
- within 14 days of stopping an MAOI intended to treat psychiatric disorders. The use of MAOIs intended to treat psychiatric disorders with Pamelor or within 14 days of stopping treatment with Pamelor is also contraindicated because of an increased risk of serotonin syndrome.
- in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue because of an increased risk of serotonin syndrome.
- in patients with hypersensitivity to tricyclic antidepressants. Cross-sensitivity between Pamelor and other dibenzazepines is a possibility.
- during the acute recovery period after a myocardial infarction.
WARNINGS and PRECAUTIONS
- All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
- The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric.
- Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.
- Prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder. Nortriptyline hydrochloride is not approved for use in treating bipolar depression.
- Patients with cardiovascular disease should be given Pamelor only under close supervision because of the tendency of the drug to produce sinus tachycardia and to prolong the conduction time. Myocardial infarction, arrhythmia, and strokes have occurred. The antihypertensive action of guanethidine and similar agents may be blocked by Pamelor.
- Pamelor should be used with great caution in patients who have a history of urinary retention due to its anticholinergic activity.
- Patients with a history of seizures should be followed closely when Pamelor is administered. This drug is known to lower the convulsive threshold.
- Cardiac arrhythmias may develop in patients with hyperthyroidism or those receiving thyroid medications.
- Pamelor may impair the mental and/or physical abilities required for the performance of hazardous tasks, such as operating machinery or driving a car; therefore, the patient should be warned accordingly.
- Excessive consumption of alcohol in combination with nortriptyline therapy may have a potentiating effect, which may lead to the danger of increased suicidal attempts or overdosage, especially in patients with histories of emotional disturbances or suicidal ideation. Deaths may occur from overdosage with this class of drugs.
- The concomitant administration of quinidine and nortriptyline may result in a significantly longer plasma half-life, higher AUC, and lower clearance of nortriptyline.
- The development of a potentially life-threatening serotonin syndrome has been reported with SNRIs and SSRIs, including Pamelor, alone but particularly with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John's Wort) and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). Treatment with Pamelor and any concomitant serotonergic agents should be discontinued immediately if serotonin syndrome occurs and supportive symptomatic treatment should be initiated.
- The use of Pamelor in schizophrenic patients may result in an exacerbation of the psychosis or may activate latent schizophrenic symptoms.
- If Pamelor is given to overactive or agitated patients, increased anxiety and agitation may occur. Troublesome patient hostility may be aroused by the use of Pamelor.
- Pamelor may increase the hazards of electroconvulsive therapy.
- Discontinue Pamelor for several days, if possible, prior to elective surgery.
- Both elevation and lowering of blood sugar levels have been reported.
- There have been postmarketing reports of an association between treatment with Pamelor and the unmasking of Brugada syndrome, a disorder characterized by syncope, abnormal electrocardiographic (ECG) findings, and a risk of sudden death. Pamelor should generally be avoided in patients with Brugada syndrome or those suspected of having Brugada syndrome.
- The pupillary dilation that occurs following use of many antidepressant drugs including Pamelor may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy.
- Safe use of Pamelor during pregnancy and lactation has not been established; therefore the potential benefits must be weighed against the possible hazards.
ADVERSE REACTIONS
The following list includes a few adverse reactions that have not been reported with Pamelor. However, the pharmacologic similarities among the tricyclic antidepressant drugs require that each of the reactions be considered when nortriptyline is administered:
Hypotension, hypertension, tachycardia, palpitation, myocardial infarction, arrhythmias, heart block, stroke, confusional states (especially in the elderly) with hallucinations, disorientation, delusions, anxiety, restlessness, agitation, insomnia, panic, nightmares, hypomania, exacerbation of psychosis, numbness, tingling, paresthesias of extremities, incoordination, ataxia, tremors, peripheral neuropathy, extrapyramidal symptoms, seizures, alteration in EEG patterns, tinnitus, dry mouth and, rarely, associated sublingual adenitis, blurred vision, disturbance of accommodation, mydriasis, constipation, paralytic ileus, urinary retention, delayed micturition, dilation of the urinary tract, skin rash, petechiae, urticaria, itching, photosensitization, edema (general or of face and tongue), drug fever, cross-sensitivity with other tricyclic drugs, bone marrow depression including agranulocytosis, eosinophilia, purpura, thrombocytopenia, nausea and vomiting, anorexia, epigastric distress, diarrhea, peculiar taste, stomatitis, abdominal cramps, black tongue, gynecomastia in the male, breast enlargement and galactorrhea in the female, increased or decreased libido, impotence, testicular swelling, elevation or depression of blood sugar levels, syndrome of inappropriate ADH (antidiuretic hormone) secretion, jaundice (simulating obstructive), altered liver function, weight gain or loss, perspiration, flushing, urinary frequency, nocturia, drowsiness, dizziness, weakness, fatigue, headache, parotid swelling and alopecia.
This is not a complete list of potential adverse events associated with Pamelor. Please see Full Prescribing Information for a complete list.
| Description | Light Orange |
| Generic Name | nortriptyline hydrochloride capsules, USP |
| Dosage Strength | 75 mg |
| Identification Code | Light orange opaque cap printed with "PAMELOR 75 mg" in black and light orange opaque body printed "M" in a box in black. |
ORDER INFORMATION
| NDC # | Package Size | Case Quantity |
|---|---|---|
| 0406-9913-03 | 30's | 6 |
For additional information on Pamelor™ (nortriptyline HCl) Capsules, USP 75 mg, call Customer Service at 1.800.325.8888 or Medical Information at 1.800.778.7898.
Code: 10004848
Revised 05/2019